Use the micropipette to transfer 25 mL of the test solution to the first well and mix. This is the first two-fold dilution. Use the micropipette with the same tip to carry out a second two-fold dilution. Continue the series of two-fold dilutions until the second last well of the microwell plate. A serial dilution is a kind of solution dilution. A more exact serial dilution definition is that it is a stepwise dilution of a solution, that is repeated a certain number of times and in which the concentration decreases with each step. The dilution factor calculator at each step does not have to be constant, but it is for this calculator.
Example #3: You have prepared 10 mL of a stock solution of 1.000 M hydrochloric acid, HCl. You serially dilute the stock solution 3 times, such that each step of the serial dilution dilutes the original solution by 1/2. A serial dilution is the stepwise dilution of a substance in solution. Usually the dilution factor at each step is constant, resulting in a geometric progression of the concentration in a logarithmic fashion. A ten-fold serial dilution could be 1 M, 0.1 M, 0.01 M, 0.001 M.
Introduction
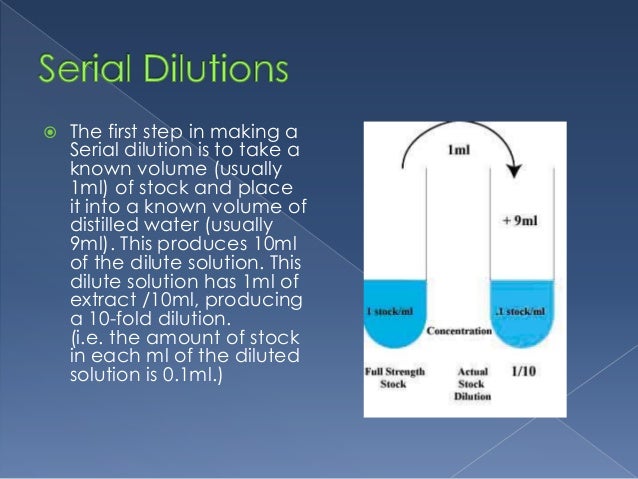
A serial dilution is a series of dilutions made sequentially, using the same dilution factor for each step. The concentration factor is the initial volume divided by the final solution volume; the dilution factor would be the inverse of the concentration factor. For example, if you take 1 part of a sample and add 9 parts of water (solvent), then you have made a 1:10 dilution; this is 1/10th (0.1) of the concentration of the original solution and has a dilution factor of 10. These serial dilutions are often used to determine the approximate concentration of an enzyme (or molecule) to be quantified in an assay. Serial dilutions allow for small aliquots to be diluted instead of wasting large quantities of materials, are cost-effective, and are easy to prepare.
Equation 1.
[concentration factor= frac{volume_{initial}}{volume_{final}}nonumber]
[dilution factor= frac{1}{concentration factor}nonumber]
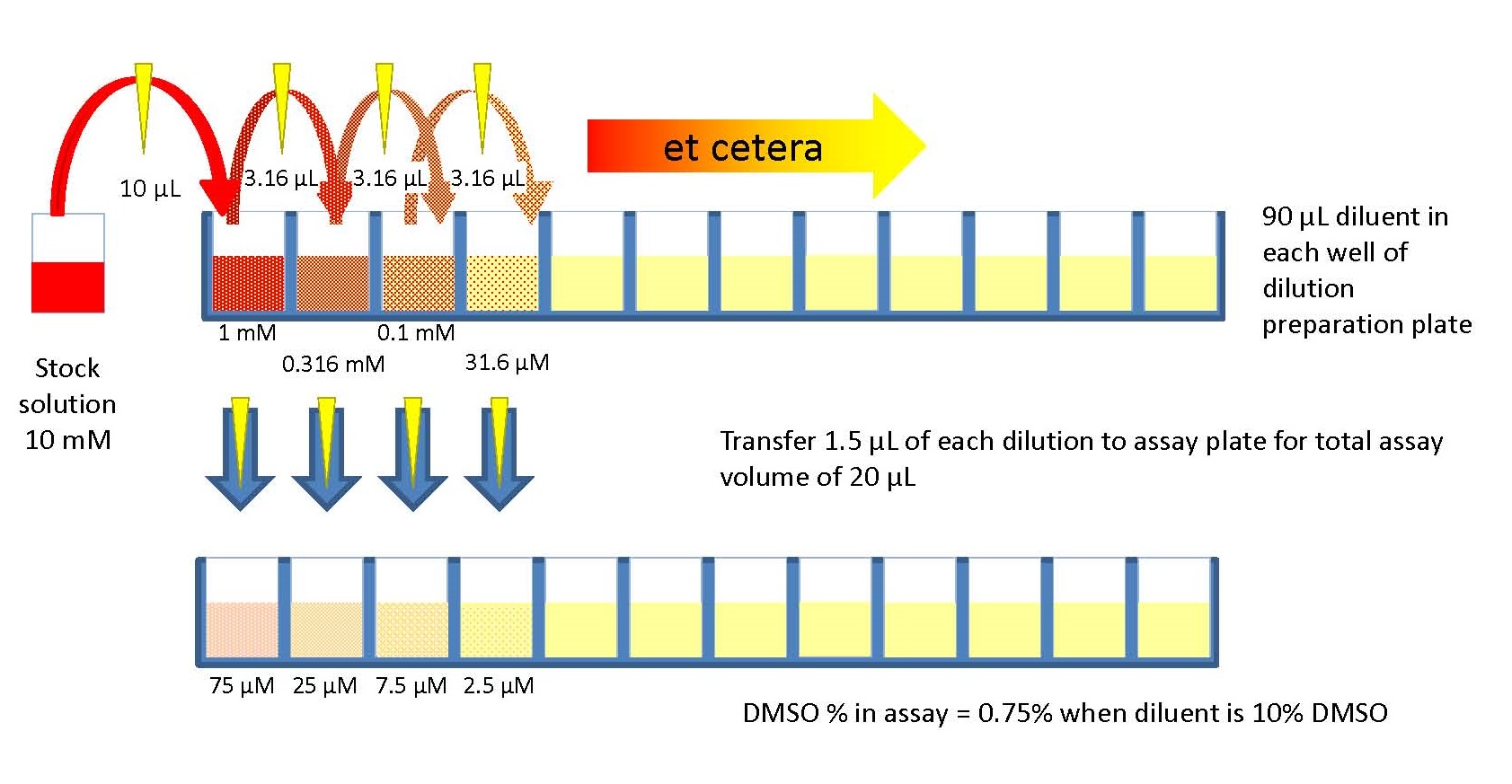
Diagram of 1:2 Serial Dilutions
In your notebook, draw a diagram showing the serial dilutions for the 6 KMnO4 solutions you are preparing. In the diagram, indicate the volume being withdrawn from the concentrated solution, the volume of water added, the concentration of the new solution, and the total volume.
Dilution Series Steps
Now that you are very comfortable with dilution factors... what happens if you dilute and then dilute again? For example, we could take super-strong cofee, dilute it by 1/5, then dilute THAT by 1/10. So,

Here's the rule:
Serial Dilution Steps Diagram
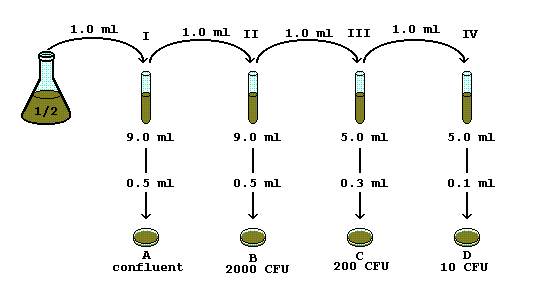
Serial Dilution Steps
To find the overall (or total) dilution factor,
simply multiply the dilution factors for each step.
Once again, here's an applet to practice finding the total dilution scheme, regardless of how the dilution scheme is expressed -- as directions, as fractions, or as decimals.
Remember, keep going until you're very comfortable with the calculations... Oh, and do yourself a favor, use a calculator!!
Given the following series of dilutions,
what is the total dilution factor?
- | | |
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Serial Dilution Steps Explained
Please do not copy without permission
How To Serial Dilute
requests/questions/feedback email: mathbench@umd.edu